Chlorophyll a
| |
| Names | |
|---|---|
| IUPAC name
Chlorophyll a
| |
| Systematic IUPAC name
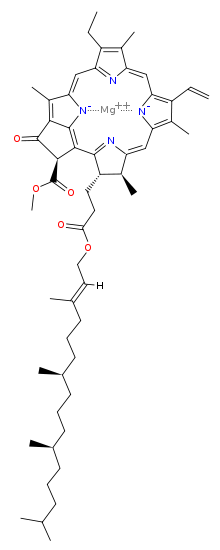
Magnesium [methyl (3S,4S,21R)-14-ethyl-4,8,13,18-tetramethyl-20-oxo-3-(3-oxo-3-{[(2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-yl]oxy}propyl)-9-vinyl-21-phorbinecarboxylatato(2−)-κ2N,N′] | |
| Other names
α-Chlorophyll
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.852 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C55H72MgN4O5 | |
| Molar mass | 893.509 g·mol−1 |
| Appearance | Green |
| Odor | Odorless |
| Density | 1.079 g/cm3[1] |
| Melting point | ~ 152.3 °C (306.1 °F; 425.4 K)[2] decomposes[1] |
| Insoluble | |
| Solubility | Very soluble in ethanol, ether Soluble in ligroin,[2] acetone, benzene, chloroform[1] |
| Absorbance | See text |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorophyll a is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light, and it is a poor absorber of green and near-green portions of the spectrum.[3] Chlorophyll does not reflect light but chlorophyll-containing tissues appear green because green light is diffusively reflected by structures like cell walls.[4] This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain.[5] Chlorophyll a also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located.[6]
Distribution of chlorophyll a
[edit]Chlorophyll a is essential for most photosynthetic organisms to release chemical energy but is not the only pigment that can be used for photosynthesis. All oxygenic photosynthetic organisms use chlorophyll a, but differ in accessory pigments like chlorophyll b.[5] Chlorophyll a can also be found in very small quantities in the green sulfur bacteria, an anaerobic photoautotroph.[7] These organisms use bacteriochlorophyll and some chlorophyll a but do not produce oxygen.[7] Anoxygenic photosynthesis is the term applied to this process, unlike oxygenic photosynthesis where oxygen is produced during the light reactions of photosynthesis.
Molecular structure
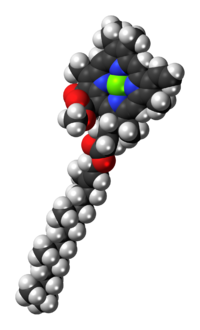
[edit]The molecular structure of chlorophyll a consists of a chlorin ring, whose four nitrogen atoms surround a central magnesium atom, and has several other attached side chains and a hydrocarbon tail formed by a phytol ester.
 |
|
| Structure of chlorophyll a molecule showing the phytol tail | |
Chlorin ring
[edit]
Chlorophyll a contains a magnesium ion encased in a large ring structure known as a chlorin. The chlorin ring is a heterocyclic compound derived from pyrrole. Four nitrogen atoms from the chlorin surround and bind the magnesium atom. The magnesium center uniquely defines the structure as a chlorophyll molecule.[8] The porphyrin ring of bacteriochlorophyll is saturated, and lacking alternation of double and single bonds causing variation in absorption of light.[9]
Side chains
[edit]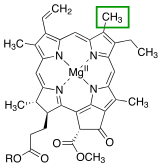
Side chains are attached to the chlorin ring of the various chlorophyll molecules. Different side chains characterize each type of chlorophyll molecule, and alters the absorption spectrum of light.[10] [11] For instance, the only difference between chlorophyll a and chlorophyll b is that chlorophyll b has an aldehyde instead of a methyl group at the C-7 position.[11]
Hydrocarbon tail
[edit]The phytol ester of chlorophyll a (R in the diagram) is a long hydrophobic tail which anchors the molecule to other hydrophobic proteins in the thylakoid membrane of the chloroplast.[5] Once detached from the porphyrin ring, phytol becomes the precursor of two biomarkers, pristane and phytane, which are important in the study of geochemistry and the determination of petroleum sources.[12]
Biosynthesis
[edit]The Chlorophyll a biosynthetic pathway utilizes a variety of enzymes.[13] In most plants, chlorophyll is derived from glutamate and is synthesised along a branched pathway that is shared with heme and siroheme.[14][15][16] The initial steps incorporate glutamic acid into 5-aminolevulinic acid (ALA); two molecules of ALA are then reduced to porphobilinogen (PBG), and four molecules of PBG are coupled, forming protoporphyrin IX.[8]
Chlorophyll synthase[17] is the enzyme that completes the biosynthesis of chlorophyll a[18][19] by catalysing the reaction EC 2.5.1.62
- chlorophyllide a + phytyl diphosphate chlorophyll a + diphosphate
This forms an ester of the carboxylic acid group in chlorophyllide a with the 20-carbon diterpene alcohol phytol.
Reactions of photosynthesis
[edit]Absorbance of light
[edit]Light spectrum
[edit]
Chlorophyll a absorbs light within the violet, blue and red wavelengths. Accessory photosynthetic pigments broaden the spectrum of light absorbed, increasing the range of wavelengths that can be used in photosynthesis.[5] The addition of chlorophyll b next to chlorophyll a extends the absorption spectrum. In low light conditions, plants produce a greater ratio of chlorophyll b to chlorophyll a molecules, increasing photosynthetic yield.[10]
Light gathering
[edit]
Absorption of light by photosynthetic pigments converts photons into chemical energy. Light energy radiating onto the chloroplast strikes the pigments in the thylakoid membrane and excites their electrons. Since the chlorophyll a molecules only capture certain wavelengths, organisms may use accessory pigments to capture a wider range of light energy shown as the yellow circles.[6] It then transfers captured light from one pigment to the next as resonance energy, passing energy one pigment to the other until reaching the special chlorophyll a molecules in the reaction center.[10] These special chlorophyll a molecules are located in both photosystem II and photosystem I. They are known as P680 for Photosystem II and P700 for Photosystem I.[20] P680 and P700 are the primary electron donors to the electron transport chain. These two systems are different in their redox potentials for one-electron oxidation. The Em for P700 is approximately 500mV, while the Em for P680 is approximately 1,100-1,200 mV.[20]
Primary electron donation
[edit]Chlorophyll a is very important in the energy phase of photosynthesis. Two electrons need to be passed to an electron acceptor for the process of photosynthesis to proceed.[5] Within the reaction centers of both photosystems there are a pair of chlorophyll a molecules that pass electrons on to the transport chain through redox reactions.[20]
Ocean
[edit]The concentration of chlorophyll A is used as an index of phytoplankton biomass. In the ocean, phytoplankton all contain the chlorophyll pigment, which has a greenish color.
Phytoplankton are microscopic organisms that live in watery environments and changes in the amount of phytoplankton indicate the change in productivity of the ocean. Phytoplankton can be affected indirectly by climatic factors, such as changes in water temperatures and surface winds.[21]
See also
[edit]- Photosystem II light harvesting protein
- Chlorophyll b, another related chemical
- Chlorophyll c, an accessory pigment of chlorophyll
References
[edit]- ^ a b c Anatolievich KR. "Chlorophyll a". chemister.ru. Archived from the original on 2014-11-29. Retrieved 2014-08-23.
- ^ a b Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ "Photosynthesis". Archived from the original on 2009-11-28.
- ^ Virtanen O, Constantinidou E, Tyystjärvi E (December 2020). "Chlorophyll does not reflect green light - how to correct a misconception". Journal of Biological Education. 56 (5): 552–559. doi:10.1080/00219266.2020.1858930.
- ^ a b c d e Raven PH, Evert RF, Eichhorn SE (2005). "Photosynthesis, Light, and Life". Biology of Plants (7th ed.). W. H. Freeman. pp. 119–127. ISBN 0-7167-9811-5.
- ^ a b Papageorgiou G, Govindjee (2004). Chlorophyll a Fluorescence, A Signature of Photosynthesis. Advances in Photosynthesis and Respiration. Vol. 19. Springer. pp. 14, 48, 86.
- ^ a b Eisen JA, Nelson KE, Paulsen IT, Heidelberg JF, Wu M, Dodson RJ, et al. (July 2002). "The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium". Proceedings of the National Academy of Sciences of the United States of America. 99 (14): 9509–14. Bibcode:2002PNAS...99.9509E. doi:10.1073/pnas.132181499. PMC 123171. PMID 12093901.
- ^ a b Zeiger E, Taiz L (2006). "Ch. 7: Topic 7.11: Chlorophyll Biosynthesis". Plant physiology (4th ed.). Sunderland, MA: Sinauer Associates. ISBN 0-87893-856-7. Archived from the original on 2020-08-07. Retrieved 2010-05-05.
- ^ Campbell MK, Farrell SO (20 November 2007). Biochemistry (6th ed.). Cengage Learning. p. 647. ISBN 978-0-495-39041-1.
- ^ a b c Lange L, Nobel P, Osmond C, Ziegler H (1981). Physiological Plant Ecology I – Responses to the Physical Environment. Vol. 12A. Springer-Verlag. pp. 67, 259.
- ^ a b Niedzwiedzki DM, Blankenship RE (December 2010). "Singlet and triplet excited state properties of natural chlorophylls and bacteriochlorophylls". Photosynthesis Research. 106 (3): 227–38. doi:10.1007/s11120-010-9598-9. PMID 21086044. S2CID 28352285.
- ^ Eglinton, G.; S. C. Brassell; Simoneit, B. R. T.; Didyk, B. M. (March 1978). "Organic geochemical indicators of palaeoenvironmental conditions of sedimentation". Nature. 272 (5650): 216–222. Bibcode:1978Natur.272..216D. doi:10.1038/272216a0. ISSN 1476-4687. S2CID 128737515.
- ^ Suzuki JY, Bollivar DW, Bauer CE (1997). "Genetic analysis of chlorophyll biosynthesis". Annual Review of Genetics. 31 (1): 61–89. doi:10.1146/annurev.genet.31.1.61. PMID 9442890.
- ^ Battersby, A. R. (2000). "Tetrapyrroles: the Pigments of Life. A Millennium review". Nat. Prod. Rep. 17 (6): 507–526. doi:10.1039/B002635M. PMID 11152419.
- ^ Akhtar, M. (2007). "The Modification of Acetate and Propionate Side Chains During the Biosynthesis of Haem and Chlorophylls: Mechanistic and Stereochemical Studies". Ciba Foundation Symposium 180 - the Biosynthesis of the Tetrapyrrole Pigments. Novartis Foundation Symposia. Vol. 180. pp. 131–155. doi:10.1002/9780470514535.ch8. ISBN 9780470514535. PMID 7842850.
- ^ Willows, Robert D. (2003). "Biosynthesis of chlorophylls from protoporphyrin IX". Natural Product Reports. 20 (6): 327–341. doi:10.1039/B110549N. PMID 12828371.
- ^ Schmid, H. C.; Rassadina, V.; Oster, U.; Schoch, S.; Rüdiger, W. (2002). "Pre-Loading of Chlorophyll Synthase with Tetraprenyl Diphosphate is an Obligatory Step in Chlorophyll Biosynthesis" (PDF). Biological Chemistry. 383 (11): 1769–78. doi:10.1515/BC.2002.198. PMID 12530542. S2CID 3099209.
- ^ Eckhardt, Ulrich; Grimm, Bernhard; Hortensteiner, Stefan (2004). "Recent advances in chlorophyll biosynthesis and breakdown in higher plants". Plant Molecular Biology. 56 (1): 1–14. doi:10.1007/s11103-004-2331-3. PMID 15604725. S2CID 21174896.
- ^ Bollivar, David W. (2007). "Recent advances in chlorophyll biosynthesis". Photosynthesis Research. 90 (2): 173–194. doi:10.1007/s11120-006-9076-6. PMID 17370354. S2CID 23808539.
- ^ a b c Ishikita H, Saenger W, Biesiadka J, Loll B, Knapp EW (June 2006). "How photosynthetic reaction centers control oxidation power in chlorophyll pairs P680, P700, and P870". Proceedings of the National Academy of Sciences of the United States of America. 103 (26): 9855–60. Bibcode:2006PNAS..103.9855I. doi:10.1073/pnas.0601446103. PMC 1502543. PMID 16788069.
- ^ "Nauru Environment Data Portal | Environmental Information for Decision Making". nauru-data.sprep.org. Retrieved 2024-01-27.
External links
[edit]- Zeiger & Taiz 2006, Topic 7.11: Chlorophyll Biosynthesis

