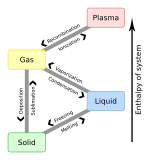
Enthalpy of sublimation
Appearance
(Redirected from Heat of sublimation)
This article needs additional citations for verification. (January 2021) |
In thermodynamics, the enthalpy of sublimation, or heat of sublimation, is the heat required to sublimate (change from solid to gas) one mole of a substance at a given combination of temperature and pressure, usually standard temperature and pressure (STP). It is equal to the cohesive energy of the solid. For elemental metals, it is also equal to the standard enthalpy of formation of the gaseous metal atoms.[1] The heat of sublimation is usually expressed in kJ/mol, although the less customary kJ/kg is also encountered.
Sublimation enthalpies
[edit]| symbol | substances | Sublimation enthalpy (kJ/mol) |
|---|---|---|
| Li | lithium | 159[1] |
| Na | sodium | 107[1] |
| K | potassium | 89[1] |
| Rb | rubidium | 81[1] |
| Cs | caesium | 76[1] |
| Mg | magnesium | 148[1] |
| Ca | calcium | 178[1] |
| Sr | strontium | 164[1] |
| Ba | barium | 180[1] |
| Fe | iron | 416[1] |
| Ni | nickel | 430[1] |
| Cu | copper | 338[1] |
| Zn | zinc | 131[1] |
| Ag | silver | 285[1] |
| W | tungsten | 849[1] |
| Au | gold | 366[1] |
| C | graphite | 717[1] |
| C | diamond | 715[1] |
| Si | silicon | 456[1] |
| Sn | tin | 302[1] |
| Pb | lead | 195[1] |
| I2 | iodine | 62.4[2] |
| C10H8 | naphthalene | 72.9[2] |
| CO2 | carbon dioxide | 25[2] |
| H2O | water | 51.1 |
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).Principles of Modern Chemistry, Brooks Cole. Appendix D. ISBN 978-1305079113
- ^ a b c Chickos, James S.; Acree, William E. (2002). "Enthalpies of Sublimation of Organic and Organometallic Compounds. 1910–2001". Journal of Physical and Chemical Reference Data. 31 (2): 537–698. doi:10.1063/1.1475333. ISSN 0047-2689.